100% Original Rivaroxaban 366789-02-8 - Ursodeoxycholic acid 128-13-2 Digestive system Cholagogic – Neore
100% Original Rivaroxaban 366789-02-8 - Ursodeoxycholic acid 128-13-2 Digestive system Cholagogic – Neore Detail:
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Production capacity: 2000kg/month
Order(MOQ): 25kg
Lead Time: 3 Working Days
Storage condition: Stored in cool, dry place, room temperature.
Package material: drum
Package size: 25kg/drum
Safety information: Not dangerous goods
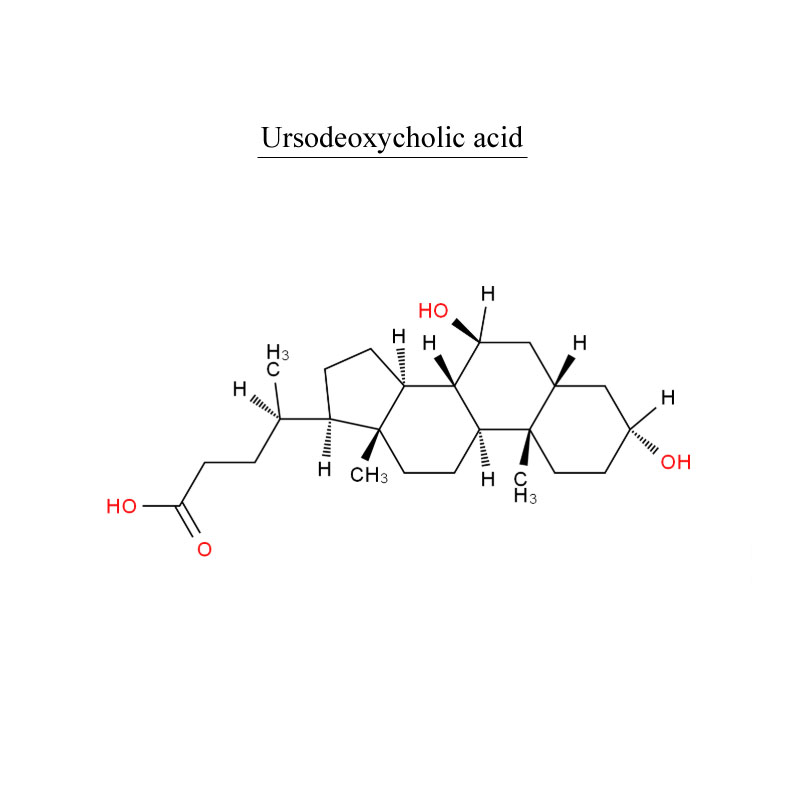
Introduction
Ursodeoxycholic acid (UDCA), also known as ursodiol, is a secondary bile acid, produced in humans and most other species from metabolism by intestinal bacteria. It is synthesized in the liver in some species, and was first identified in bear bile, which is the derivation of its name Ursus. In purified form, it has been used to treat or prevent several diseases of the liver or bile ducts.
UDCA has been used as medical therapy in gallstone disease (cholelithiasis) and for biliary sludge. UDCA helps reduce the cholesterol saturation of bile and leads to gradual dissolution of cholesterol-rich gallstones.
UDCA may be given after bariatric surgery to prevent cholelithiasis, which commonly occurs due to the rapid weight loss producing biliary cholesterol oversaturation and also biliary dyskinesia secondary hormonal changes.
Specification (EP10)
|
Item |
Specification |
|
Appearance |
White or almost white powder |
|
Solubility |
Practically insoluble in water, freely soluble in ethanol (96%), slightly soluble in acetone, practically insoluble in methylene chloride |
| Melting point |
202-204℃ |
|
Identification |
Same IR spectrum as ursodeoxycholic acid CRS |
| The principal spot in the chromatogram obtained with the test solution (b) is similar in position, colour and size to the principle spot in the chromatogram obtained with reference solution (a). | |
| The suspension obtained is greenish-blue. | |
| Specific optical rotation |
+58.0~+62.0° |
| Impurity C |
Lithocholic acid ≤ 0.1% |
| Related substance (HPLC) |
impurity A: chenodeoxycholic acid ≤ 1.0% |
|
unspecified impurities ≤ 0.1% |
|
|
total ≤ 1.5% |
|
| Heavy metals |
ICH Q3D |
|
Loss on drying |
≤ 1.0% |
| Sulphated ash |
≤ 0.1% |
| Assay |
99.0%~101% (Dried substance) |
| Residual solvents |
Acetone ≤ 5000 ppm Ethyl acetate ≤ 5000 ppm Isopropanol ≤ 5000 ppm Ethanol ≤ 5000 ppm |
| Microbiological Tests |
Total aerobic microbial count ≤ 10³CFU/g Total yeasts and moulds count ≤ 10²CFU/g Escherichia coli: Absent in 1 g Salmonella: Absent in 10 g |
| Additional properties | |
| Particle size distribution |
100% pass number 180 sieve (100% pass 80 mesh sieve) |
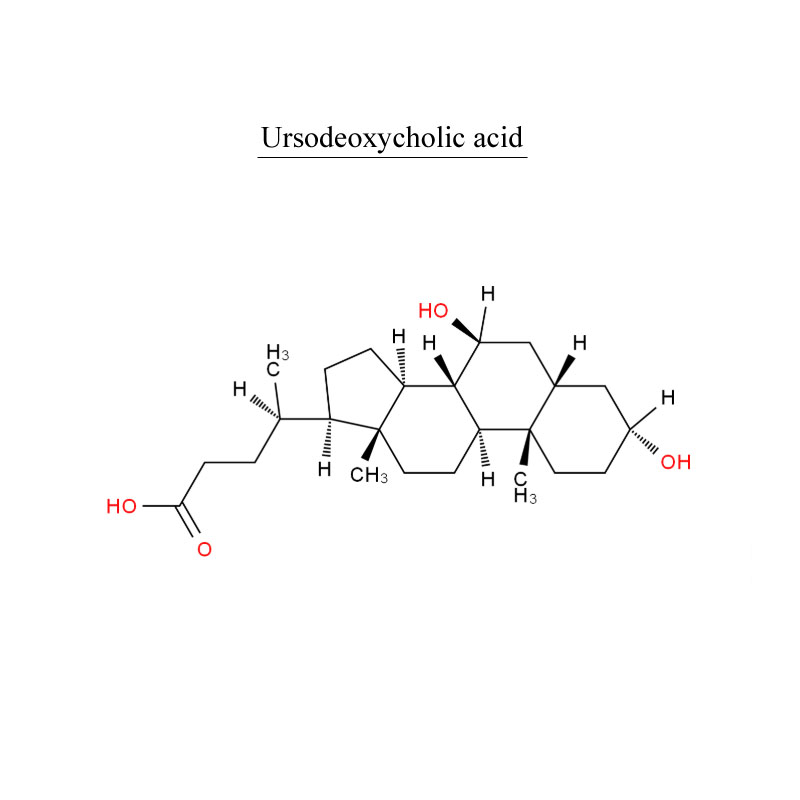
Product detail pictures:
Related Product Guide:
We've been convinced that with joint efforts, the enterprise between us will bring us mutual benefits. We are able to assure you product or service quality and aggressive cost for 100% Original Rivaroxaban 366789-02-8 - Ursodeoxycholic acid 128-13-2 Digestive system Cholagogic – Neore , The product will supply to all over the world, such as: United Kingdom, Sheffield, Manchester, Our market share of our products has greatly increased yearly. If you are interested in any of our products or would like to discuss a custom order, please feel free to contact us. We are looking forward to forming successful business relationships with new clients around the world in the near future. We are looking forward to your inquiry and order.
The factory workers have rich industry knowledge and operational experience, we learned a lot in working with them,we are extremely grateful that we can encount a good company has excellent wokers.