8 Year Exporter Latanoprost - Tacrolimus monohydrate 109581-93-3 Antibiotic – Neore
8 Year Exporter Latanoprost - Tacrolimus monohydrate 109581-93-3 Antibiotic – Neore Detail:
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Production capacity: 1kg/month
Order(MOQ): 1g
Lead Time: 3 Working Days
Storage condition: Stored in cool, dry place, room temperature.
Package material: vial, bottle
Package size: 1g/vial, 5/vial, 10g/vial, 50g/bottle, 500g/bottle
Safety information: UN 2811 6.1/PG 3
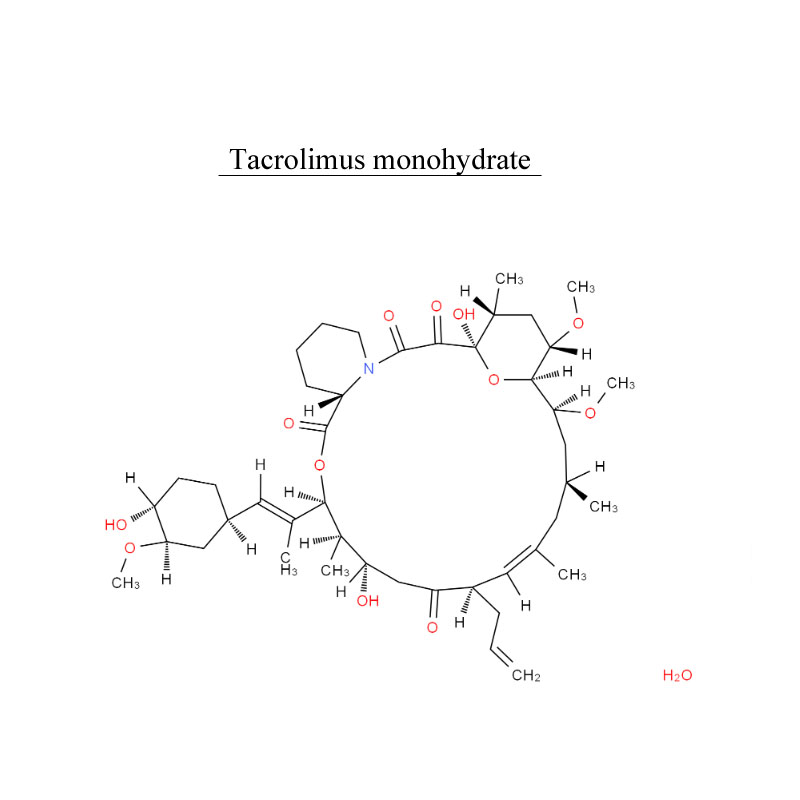
Introduction
Tacrolimus, is an immunosuppressive drug. After allogeneic organ transplant, the risk of organ rejection is moderate. To lower the risk of organ rejection, tacrolimus is given. The drug can also be sold as a topical medication in the treatment of T-cell-mediated diseases such as eczema and psoriasis. It can be used to treat dry eye syndrome in cats and dogs.
Tacrolimus inhibits calcineurin, which is involved in the production of interleukin-2, a molecule that promotes the development and proliferation of T cells, as part of the body’s learned (or adaptive) immune response.
Specification (USP43)
|
Item |
Specification |
|
Appearance |
White or almost white crystalline powder |
|
Identification |
IR, HPLC |
| Solubility |
Very soluble in methanol, freely soluble in N,N dimethylformamide and in alcohol, practically in soluble in water. |
|
Residue on ignition |
≤0.10 % |
|
Organic impurities (procedure-2) |
Ascomycin 19-epimer ≤0.10 % |
|
Ascomycin ≤0.50 % |
|
|
Desmethyl tacrolimus ≤0.10 % |
|
|
Tacrolimus 8-epimer ≤0.15 % |
|
|
Tacrolimus 8-propyl analog ≤0.15 % |
|
|
Unknown impurity -I ≤0.10 % |
|
|
Unknown impurity -II ≤0.10 % |
|
|
Unknown impurity -III ≤0.10 % |
|
|
Total impurities ≤1.00 % |
|
|
Optical rotation (on as is basis) (10mg/ml in N,Ndimethylformamide) |
-110.0° ~ -115.0° |
|
Water content (by KF) |
≤4.0% |
|
Residual solvents (by GC) |
Acetone ≤1000ppm (In-house) |
|
Di-isopropyl ether ≤100ppm (In-house) |
|
|
Ethyl ether ≤5000ppm |
|
|
Acetonitrile ≤410ppm |
|
|
Toluene ≤890ppm |
|
|
Hexane ≤290ppm |
|
|
Microbial test (in house) |
Total aerobic microbial count ≤100cfu/gm |
|
Total yeast and mould count ≤10cfu/gm |
|
|
Specified organisms (Pathogens) (E.coil, salmonella sps., S.aureus. Pseudomonas aeruginosa) should absent |
|
|
Assay (by HPLC) (on anhydrous and solvent free basis) |
98%~102% |
Product detail pictures:
Related Product Guide:
Persisting in "High quality, Prompt Delivery, Aggressive Price", now we have established long-term cooperation with consumers from equally overseas and domestically and get new and old clients' large comments for 8 Year Exporter Latanoprost - Tacrolimus monohydrate 109581-93-3 Antibiotic – Neore , The product will supply to all over the world, such as: Tanzania, Danish, UAE, Custom orders are acceptable with different quality grade and customer's especial design. We are looking forward to establish the good and successful cooperation in business with long terms from the customers of all over the world.
Factory equipment is advanced in the industry and the product is fine workmanship, moreover the price is very cheap, value for money!