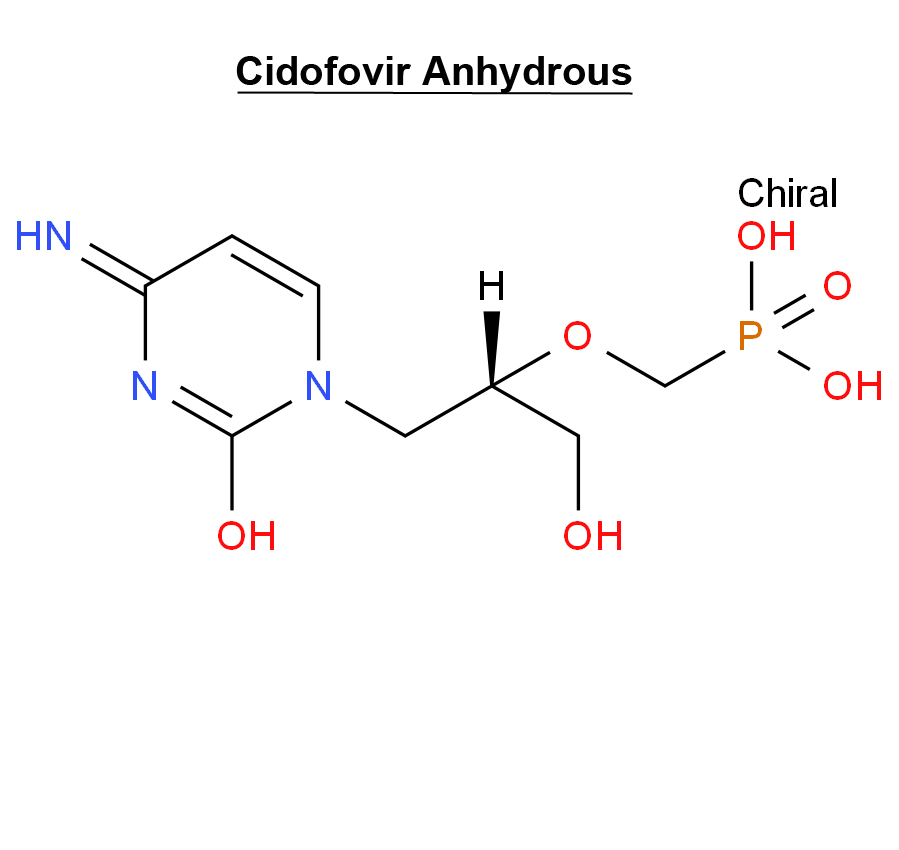
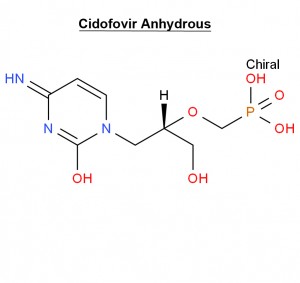
Cidofovir Anhydrous 113852-37-2 Antiviral
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Production capacity: 1kg/month
Order(MOQ): 1g
Lead Time: 3 Working Days
Storage condition: Stored in cool and dry place, sealed and keep away from light.
Package material:vial, bottle
Package size:1g/vial, 5/vial, 10g/vial, 50g/bottle, 500g/bottle
Safety information: UN 2811 6.1/ PG 3
Introduction
Cidofovir dihydrate is the dihydrate of the anhydrous form of cidofovir. A nucleoside analogue, it is an injectable antiviral used for the treatment of cytomegalovirus (CMV) retinitis in AIDS patients. It has a role as an antiviral drug and an antineoplastic agent.
Specification (in house standard)
|
Items |
Specifications |
|
Appearance |
White to off white crystalline powder |
|
Identification
|
Infrared Spectroscopy |
| The retention time of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. | |
|
Residue on Ignition |
≤0.5% |
|
Organic Impurities
|
Cidofovir diol analoga ≤0.15% |
|
Cidofovir related compound A ≤0.15% |
|
|
Cidofovir related compound B ≤0.15% |
|
|
Cidofovir uracil analogb ≤0.15% |
|
|
Bromocidofovirc ≤0.15% |
|
|
Any individual unspecified impurity ≤0.10% |
|
|
Total impurities ≤1.0% |
|
|
Enantiomeric Purity |
≤1.0% |
| Microbial Enumeration Testsand Tests for Specified Microorganisms | The total aerobic bacterial count is NMT 102 cfu/g. The total combined molds and yeasts count is NMT 101 cfu/g. |
|
Water Determination |
≤0.5% |
|
pH |
2.5-4.5 |
|
Assay |
98.0%-102.0%, on the anhydrous basis |