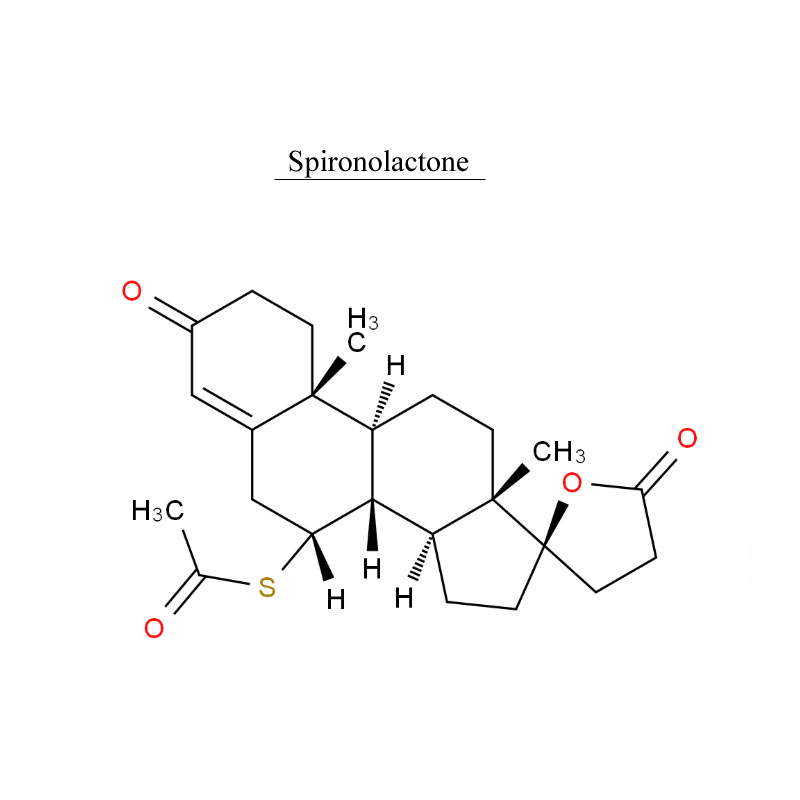
Factory making 3-O-Ethyl Ascorbic Acid 86404-04-8 - Spironolactone 52-01-7 Urinary system – Neore
Factory making 3-O-Ethyl Ascorbic Acid 86404-04-8 - Spironolactone 52-01-7 Urinary system – Neore Detail:
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Production capacity: 50kg/month
Order(MOQ): 25kg
Lead Time: 3 Working Days
Storage condition: Stored in cool, dry place, room temperature.
Package material: drum
Package size: 25kg/drum
Safety information: Not dangerous goods
Introduction
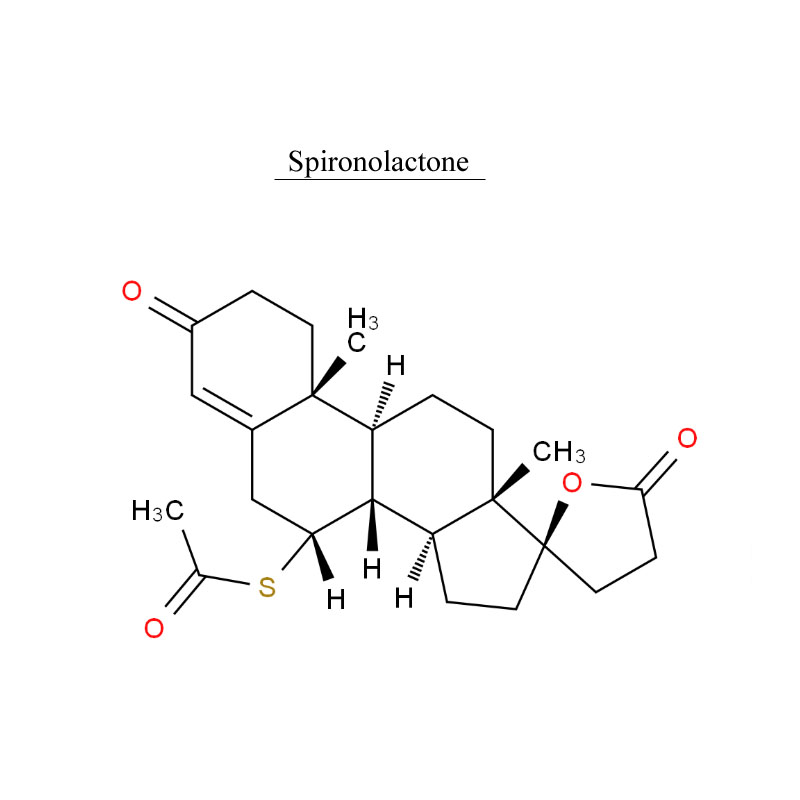
Spironolactone, is a medication that is primarily used to treat fluid build-up due to heart failure, liver scarring, or kidney disease. It is also used in the treatment of high blood pressure, low blood potassium that does not improve with supplementation, early puberty in boys, acne and excessive hair growth in women, and as a part of transgender hormone therapy in transfeminine people.
Specification (USP42)
|
Item |
Specification |
|
Appearance |
Light cream-colored to light tan crystalline powder. |
|
Solubility (annual) |
Freely soluble in benzene and in chloroform; soluble in ethyl acetate and in alcohol; slightly soluble in methanol and in fiexed oils; practically insoluble in water. |
|
Identification |
Infrared Absorption: meets the requirement |
|
HPLC: meets the requirement |
|
|
Limit of mercapto compounds |
≤0.10mL of 0.010N iodine is consumed |
|
Organic impurities |
Related compound B ≤0.2% |
|
Related compound A ≤0.2% |
|
|
Related compound C ≤0.2% |
|
|
Related compound D ≤0.3% |
|
|
Epimer ≤0.3% |
|
|
Related compound I ≤0.1% |
|
|
Any unspecified impurity ≤0.10% |
|
|
Total impurities ≤1.0% |
|
| Optical rotation | -41°~ -45° |
| Loss on drying | ≤0.5% |
| Residual solvents (In-house) | Methanol ≤3000ppm |
| Tetrahydrofuran ≤720ppm | |
| DMF ≤880ppm | |
| Particle size (In-house) | 95% not more than 20 microns |
|
Assay |
97.0% ~103.0% on the dried basis |
Product detail pictures:
Related Product Guide:
Our target is to consolidate and improve the quality and service of existing products, meanwhile constantly develop new products to meet different customers' demands for Factory making 3-O-Ethyl Ascorbic Acid 86404-04-8 - Spironolactone 52-01-7 Urinary system – Neore , The product will supply to all over the world, such as: Guatemala, Morocco, Congo, Establish long term and win-win business relationships with all our customers, share the success and enjoy the happiness of spreading our products to the world together. Trust us and you will gain more. Please feel free to contact us for more information, we assure you of our best attention at all times.
Company director has very rich management experience and strict attitude, sales staff are warm and cheerful, technical staff are professional and responsible,so we have no worry about product,a nice manufacturer.