Manufacturer for 501-30-4 - Tacrolimus monohydrate 109581-93-3 Antibiotic – Neore
Manufacturer for 501-30-4 - Tacrolimus monohydrate 109581-93-3 Antibiotic – Neore Detail:
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Production capacity: 1kg/month
Order(MOQ): 1g
Lead Time: 3 Working Days
Storage condition: Stored in cool, dry place, room temperature.
Package material: vial, bottle
Package size: 1g/vial, 5/vial, 10g/vial, 50g/bottle, 500g/bottle
Safety information: UN 2811 6.1/PG 3
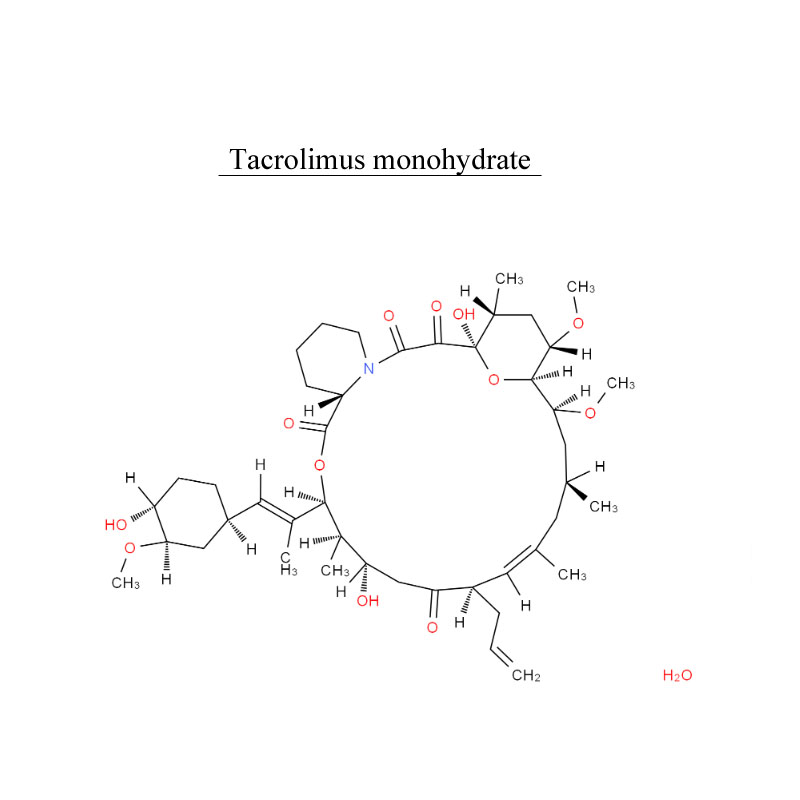
Introduction
Tacrolimus, is an immunosuppressive drug. After allogeneic organ transplant, the risk of organ rejection is moderate. To lower the risk of organ rejection, tacrolimus is given. The drug can also be sold as a topical medication in the treatment of T-cell-mediated diseases such as eczema and psoriasis. It can be used to treat dry eye syndrome in cats and dogs.
Tacrolimus inhibits calcineurin, which is involved in the production of interleukin-2, a molecule that promotes the development and proliferation of T cells, as part of the body’s learned (or adaptive) immune response.
Specification (USP43)
|
Item |
Specification |
|
Appearance |
White or almost white crystalline powder |
|
Identification |
IR, HPLC |
| Solubility |
Very soluble in methanol, freely soluble in N,N dimethylformamide and in alcohol, practically in soluble in water. |
|
Residue on ignition |
≤0.10 % |
|
Organic impurities (procedure-2) |
Ascomycin 19-epimer ≤0.10 % |
|
Ascomycin ≤0.50 % |
|
|
Desmethyl tacrolimus ≤0.10 % |
|
|
Tacrolimus 8-epimer ≤0.15 % |
|
|
Tacrolimus 8-propyl analog ≤0.15 % |
|
|
Unknown impurity -I ≤0.10 % |
|
|
Unknown impurity -II ≤0.10 % |
|
|
Unknown impurity -III ≤0.10 % |
|
|
Total impurities ≤1.00 % |
|
|
Optical rotation (on as is basis) (10mg/ml in N,Ndimethylformamide) |
-110.0° ~ -115.0° |
|
Water content (by KF) |
≤4.0% |
|
Residual solvents (by GC) |
Acetone ≤1000ppm (In-house) |
|
Di-isopropyl ether ≤100ppm (In-house) |
|
|
Ethyl ether ≤5000ppm |
|
|
Acetonitrile ≤410ppm |
|
|
Toluene ≤890ppm |
|
|
Hexane ≤290ppm |
|
|
Microbial test (in house) |
Total aerobic microbial count ≤100cfu/gm |
|
Total yeast and mould count ≤10cfu/gm |
|
|
Specified organisms (Pathogens) (E.coil, salmonella sps., S.aureus. Pseudomonas aeruginosa) should absent |
|
|
Assay (by HPLC) (on anhydrous and solvent free basis) |
98%~102% |
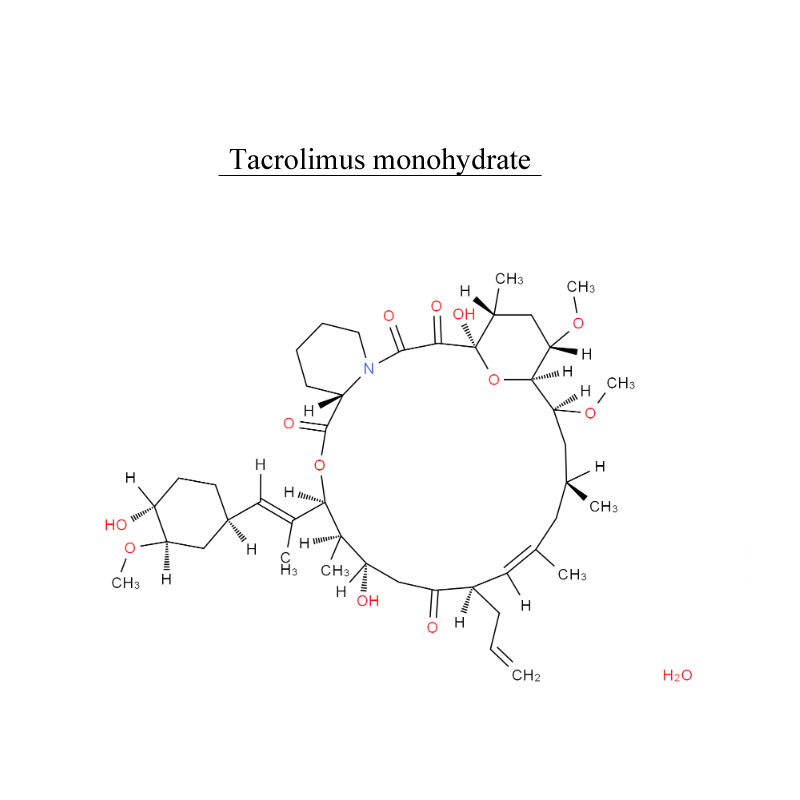
Product detail pictures:
Related Product Guide:
We strive for excellence, service the customers", hopes to become the best cooperation team and dominator enterprise for personnel, suppliers and customers, realizes value share and continuous promotion for Manufacturer for 501-30-4 - Tacrolimus monohydrate 109581-93-3 Antibiotic – Neore , The product will supply to all over the world, such as: Suriname, Chile, Saudi Arabia, Insisting on the high quality generation line management and customers expert assistance, we now have designed our resolution to supply our buyers using the to start with amount getting and just after services practical experience. Maintaining the prevailing friendly relations with our buyers, we however innovate our solution lists all of the time to satisfy the brand new demands and adhere to the most up-to-date development of the market in Malta. We have been ready to face the worries and make the improve to understand all the possibilities in international trade.
We have been engaged in this industry for many years, we appreciate the work attitude and production capacity of the company, this is a reputable and professional manufacturer.