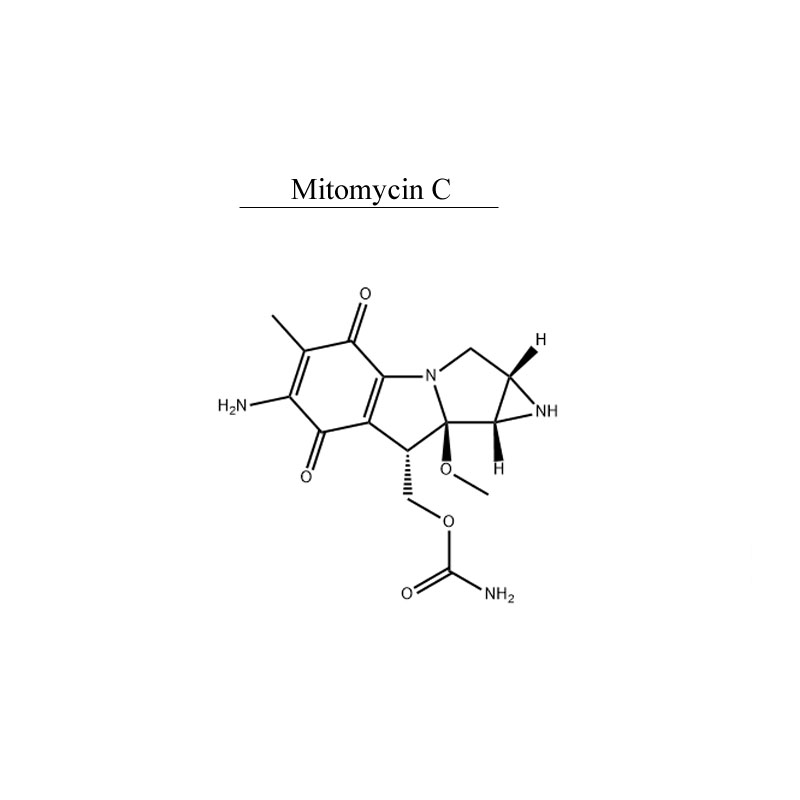
Manufacturer for Ursodeoxycholic acid - Mitomycin C 50-07-7 Antibiotic Antineoplastic – Neore
Manufacturer for Ursodeoxycholic acid - Mitomycin C 50-07-7 Antibiotic Antineoplastic – Neore Detail:
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Production capacity: 5kg/month
Order(MOQ): 10g
Lead Time: 3 Working Days
Storage condition: Stored in cool, dry place, room temperature.
Package material: vial
Package size: 10g/drum
Safety information: UN 2811 6.1/ PG 1
Description
Mitomycin C is a mitomycin that is used as a chemotherapeutic agent by virtue of its antitumour activity.
It is given intravenously to treat upper gastro-intestinal cancers (e.g. esophageal carcinoma), anal cancers, and breast cancers, as well as by bladder instillation for superficial bladder tumours.
Mitomycin C is used in cancers, particularly bladder cancers and intraperitoneal tumours.
Mitomycin C is used in eye surgery where mitomycin C 0.02% is applied topically to prevent scarring during glaucoma filtering surgery and to prevent haze after PRK or LASIK; mitomycin C has also been shown to reduce fibrosis in strabismus surgery.
Mitomycin C is used in esophageal and tracheal stenosis where application of mitomycin C onto the mucosa immediately following dilatation will decrease re-stenosis by decreasing the production of fibroblasts and scar tissue.
Specification (USP/EP)
|
Item |
Specification |
|
Appearance |
Blue-violet, crystalline powder |
|
Identification |
IR: The IR spectrum of the sample corresponds to the spectrum of reference standard |
| HPLC: The retention time of the major peak of the sample solution corresponds to that of the standard solution, as obtained in the Assay | |
| pH |
6.0~7.5 |
| Water |
Not more than 2.5% |
| Crystallinity |
Should conforms |
| Related Substances | |
| Albomitomycin C
(EP Impurity D) |
Not more than 0.5% |
| Mitomycin B
(EP Impurity C) |
Not more than 0.5% |
| Cinnamamide
(EP Impurity A) |
Not more than 0.5% |
| Mitomycin A
(EP Impurity B) |
Not more than 0.5% |
| Any Individual Unspecified Impurity |
Not more than 0.5% |
| Total Impurities |
Not more than 2.0% |
| Residual Solvents | |
| Methanol |
Not more than 3000 ppm |
| Methylene Chloride |
Not more than 600 ppm |
| Ethyl Acetate |
Not more than 5000 ppm |
| Bacterial Endotoxins |
Not more than 10 EU/mg |
| Assay |
Not less than 970 mg/g of Mitomycin |
Product detail pictures:

Related Product Guide:
Being supported by an advanced and specialist IT team, we could give technical support on pre-sales & after-sales services for Manufacturer for Ursodeoxycholic acid - Mitomycin C 50-07-7 Antibiotic Antineoplastic – Neore , The product will supply to all over the world, such as: Malaysia, European, Eindhoven, Strict quality control is executed in each link of the whole production process.We sincerely hope to establish the friendly and mutual-beneficial cooperation with you. Based on high quality products and perfect pre-sales /after-sales service is our idea, some clients had cooperated with us for more than 5 years.
The company account manager has a wealth of industry knowledge and experience, he could provide appropriate program according our needs and speak English fluently.