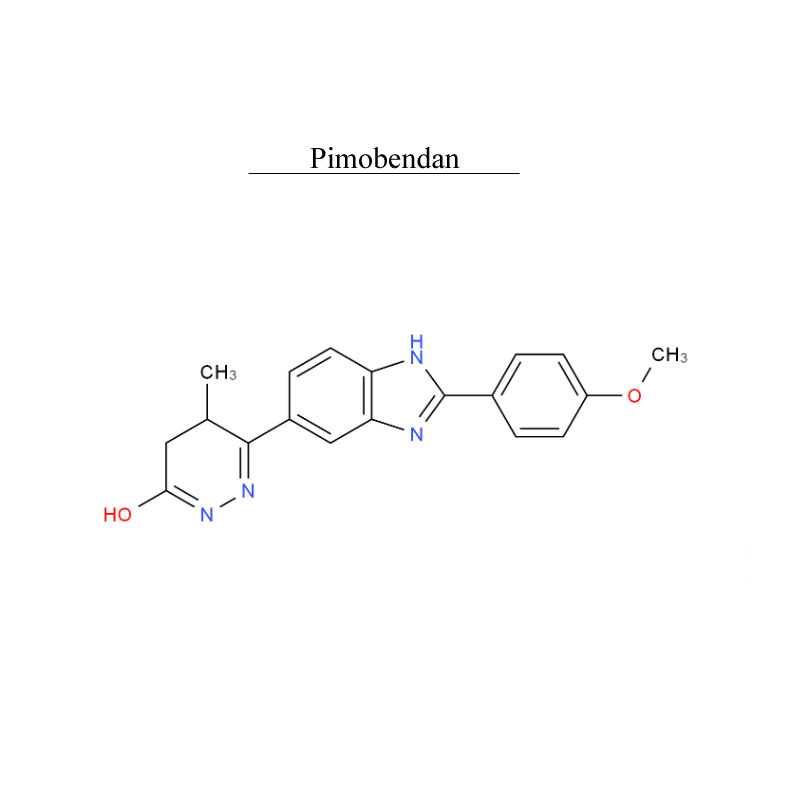
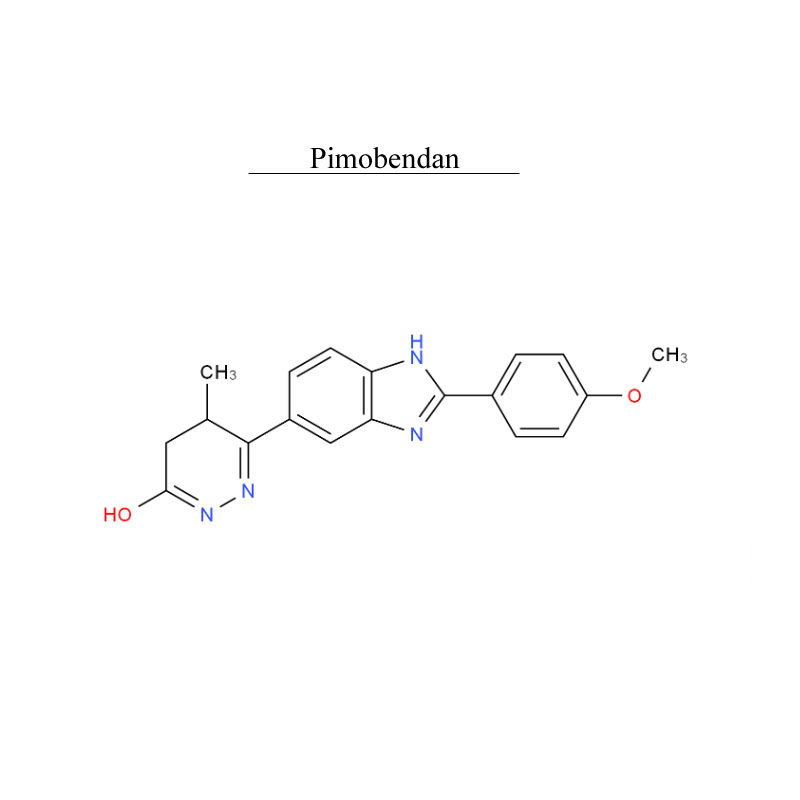
Pimobendan 74150-27-9 Metabolism PDE inhibitor
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Order (MOQ): 1g
Lead Time: 3 working days
Production capacity: 1kg/month
Storage condition: Stored in cool, dry place, room temperature.
Package material: vial, bottle
Package size: 1g/vial, 5/vial, 10g/vial, 50g/bottle, 500g/bottle
Safety information: UN 2811 6.1/PG 3
Introduction
Pimobendan, is a veterinary medication. It is a calcium sensitizer and a selective inhibitor of phosphodiesterase 3 (PDE3) with positive inotropic and vasodilator effects.
Pimobendan is used in the management of heart failure in dogs, most commonly caused by myxomatous mitral valve disease (also previously known as endocardiosis), or dilated cardiomyopathy. Research has shown that as a monotherapy, pimobendan increases survival time and improves quality of life in canine patients with congestive heart failure secondary to mitral valve disease when compared with benazepril, an ACE inhibitor.
Specification (USP43)
|
Item |
Specification |
|
Appearance |
White or slightly yellowish powder, hygroscopic |
|
Mp |
About 242℃ |
|
Solubility |
Practically insoluble in water, freely soluble in dimethylformamide, slightly soluble in acetoneand in methanil. |
|
Identification |
Infrared absorption spectrophotometry, Comparison pimobendan CRS. |
|
The retention time of the major peak of the Sample solution corresponds to that of the Standard stock solution,as obtained in the Organic Impurities test. |
|
|
Heavy metals |
≤10ppm |
|
Granularity |
P90 ≤ 25μ m |
|
Particle size |
20-80 mesh |
|
Residual solvents |
≤ 500ppm |
|
Water |
≤ 1.0% |
|
Assay |
98.0%~102.0% |
|
Sulphated ash |
≤ 0.10% |
|
Related substances (HPLC) |
|
| impurity A |
≤ 0.10% |
| impurity B |
≤ 0.10% |
| Any other impurity |
≤ 0.10% |
| Total impurity |
≤ 0.20% |