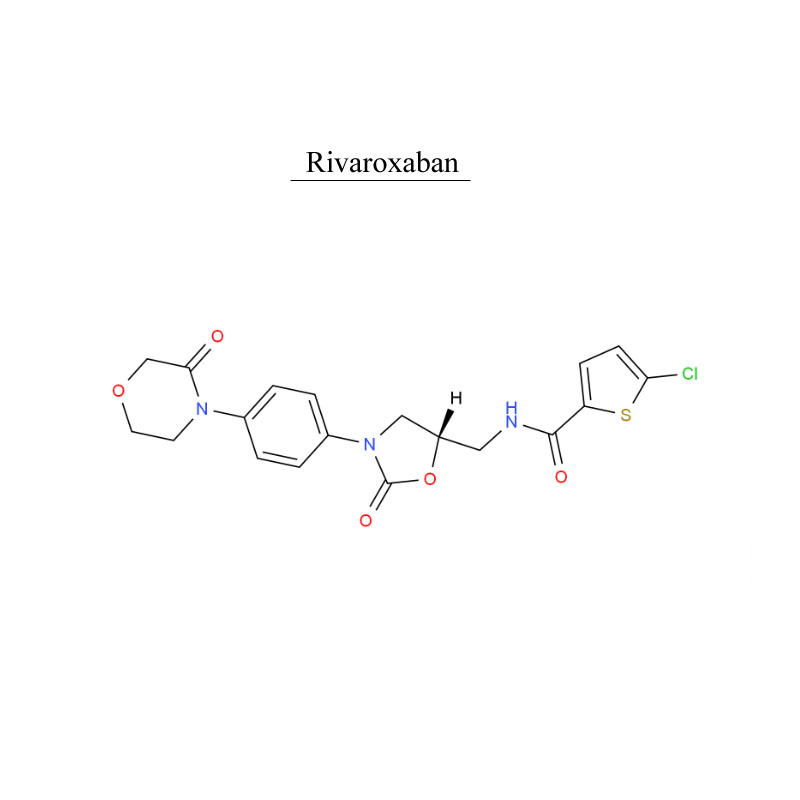
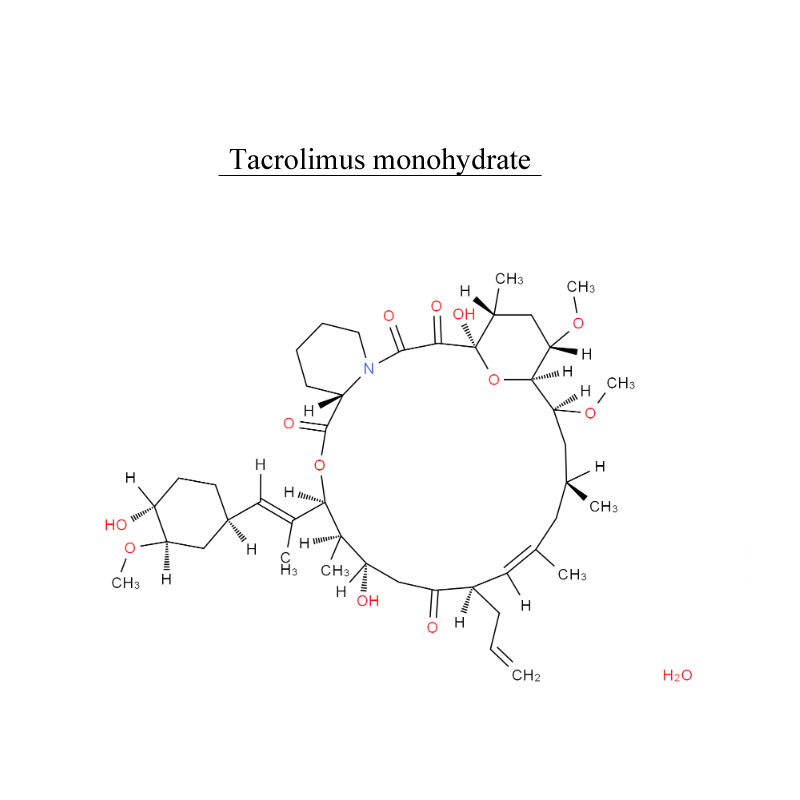
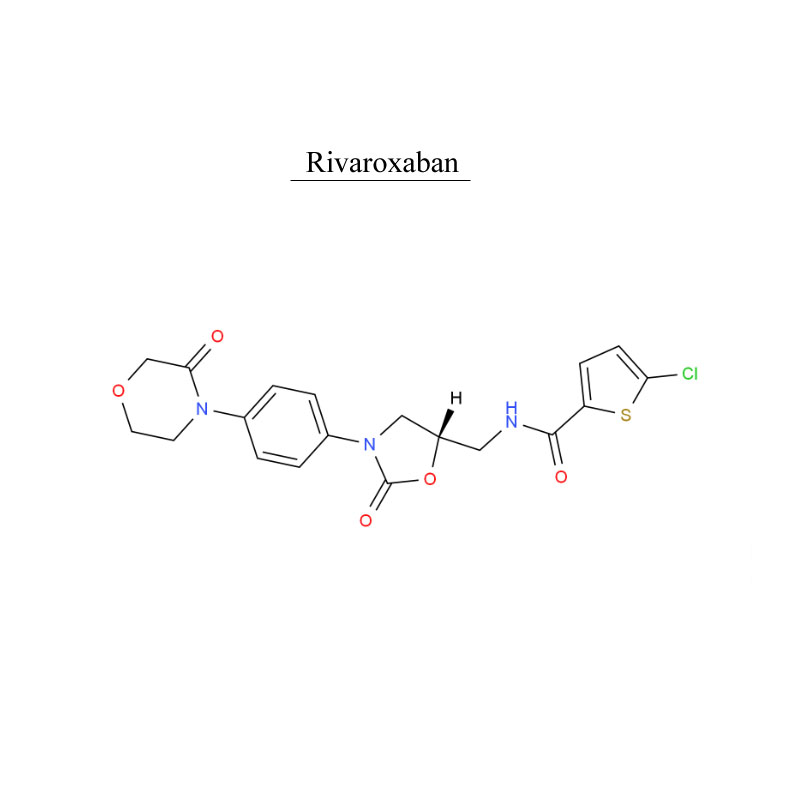
Rivaroxaban 366789-02-8 Blood system protect Antithrombosis
Payment: T/T, L/C
Product Origin: China
Shipping Port: Beijing/Shanghai/Hangzhou
Production capacity: 200kg/month
Order(MOQ): 25kg
Lead Time: 3 Working Days
Storage condition: Stored in cool, dry place, room temperature.
Package material: drum
Package size: 25kg/drum
Safety information: Not dangerous goods
Introduction
Rivaroxaban, is an anticoagulant medication (blood thinner). It use in adults with nonvalvular atrial fibrillation (except atrial fibrillation due to rheumatic valvular heart disease, and atrial fibrillation after heart valve replacement) to reduce the risk of stroke and systemic embolism.
Rivaroxaban, is used to treat and prevent blood clots.
Rivaroxaban, is used f for adult patients undergoing elective hip or knee replacement surgery to prevent venous thrombosis.
Specification (EP)
|
Item |
Specification |
|
Appearance |
White or yellowish powder |
|
Solubility |
Practically insoluble in water, freely soluble in DMSO, practically insoluble in anhydrous ethanol and in heptanes. |
|
Identification |
IR: Spectrum should comply with the WS |
|
HPLC-RT of sample under enantiometric purity test should comply with that of WS. |
|
| Water | ≤0.5% |
|
Sulphate ash |
≤0.1% |
|
Loss on drying |
≤0.5% |
| Heavy metals | ≤20ppm |
|
Enantionmer |
Impurity A: ≤0.4% |
|
Residual solvent |
Ethanol: ≤5000ppm
Ethyl acetate: ≤5000ppm Acetone: ≤5000ppm N,N-Dimethylformamide: ≤880ppm Methylene chloride: ≤600ppm Benzene: ≤2ppm |
|
Related substance |
Impurity B: ≤0.10%
Impurity D: ≤0.10% Impurity E: ≤0.10% Impurity F: ≤0.10% Impurity G: ≤0.10% Impurity H: ≤0.10% Impurity I: ≤0.10% Impurity J: ≤0.10% RVB-4-NJSH: ≤0.10% RVB-ZA: ≤0.10% RVB-BAM: ≤0.10% RVB-ZC: ≤0.10% RVB-HBY: ≤75ppm Any other unknown impurity: ≤0.10% Total impurity: ≤0.3% |
|
Particle size distribution |
D10:/
D50:/ D90:/ |
| Assay
(anhydrous substance) |
98.0-102.0 |